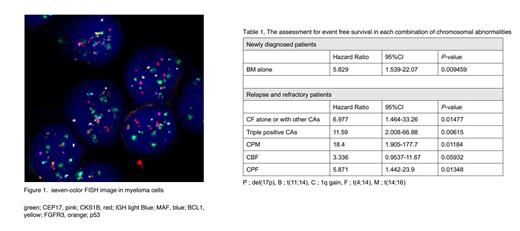
Introduction : The proliferation of heterogeneous clones in multiple myeloma (MM) cells is associated with progression and resistance to treatments. Chromosomal abnormalities (CAs) were one of the drivers of myeloma progression and resistance mechanisms for the treatment. Here, we developed the method of simultaneous detection of five CAs in single cell by seven colors-fluorescent in situ hybridization (FISH). With this FISH analysis, we analyzed the acquisition patterns of CAs and the relationships between complex CAs with treatment response and prognosis.
Methods: Patients diagnosed as MM who provided written informedconsent to participate in the study were enrolled. All the patients were treated with three or more drug-combined treatments based on proteasome inhibitors and/or IMiDs between 2014 and 2022 in our institution. Bone marrow aspiration and biopsy were performed at the time of diagnosis and upon disease progression. Myeloma cells were isolated from bone marrow samples using anti-CD138-MicroBeads. These cells were spotted on the slide glasses. After fixation and permeabilization, cells were hybridized with 7 series of probes simultaneously to detect targeted five CAs, 1q gain, del(17p), t(11;14), t(14;18), and t(4;14). The fluorescent signals were detected by confocal microscopy (ZEISS, LSM880) (Figure 1). We counted 100 or more cells in each patient and calculated the positive ratio of the five series of CAs. The relationships between each combination of CAs and non-complete response (non-CR) status were analyzed by Mann-Whitney test. The event free survival (EFS) was analyzed by Kaplan-Meier and the significant relationships were calculated by log-rank test. EFS were estimated from the start of the treatment to the days of progression, start of next treatment, or death. This protocol was approved by the institutional review board of Japanese Foundation for Cancer Research.
Result: The enrolled patients were 41 including 24 newly diagnosed (ND) and 17 relapse and refractory (RR) patients. The median age was 65.8 years (40-85) in ND patients and 70.3 years (50-85) in RR patients. In ND patients, the presence of del(17p) with t(11;14) (58%; 14/24), 1q gain with t(11;14) (50%; 12/24), and t(4;14) with t(14;16) (50%; 12/24) were frequently observed. Double positivity of t(11;14) and t(14;16) alone or in combination with other CAs and t(11;14) and del(17p) alone were associated with non-CR ( P<0.035 and P<0.041, respectively). Furthermore, patients with t(11;14) and t(14;16) alone had lower EFS (HR 5.829, 95%CI;1.539-22.07) (Table 1). In RR patients, the double positivity of del(17p) and 1q gain increased to 88% (15/17) compared to 42% (10/24) in ND patients. The double positivity of t(14;16) and t(4;14) alone or with other CAs, and triple positive CAs were associated with non-CR, ( P=0.048, and 0.037, respectively). Patients with 1q gain and t(4;14) alone or with other CAs (HR 6.977, 95%CI;1.464-33.26 ) and triple positive CAs had lower EFS (HR 7.781, 95%CI; 0.98-61.76); Among triple positive CAs, the combination of 1q gain, del(17p), and t(4;14) (HR 5.871, 95%CI; 1.442-23.9), as well as the combination of 1q gain, del(17p), and t(14;16) (HR 4.363 95%CI; 1.189-16) were related to lower EFS (Table 1).
Conclusion: The simultaneous FISH analysis in single cells is useful for detecting the acquisition-pattern of CA in myeloma. This method is also useful even in low numbers of myeloma cells. The single CA does not always correlate to the treatment response or prognosis. The detection of the combined acquisition of CAs might be provided a novel prognosis factor.
Disclosures
Mishima:Janssen: Honoraria; Takeda: Research Funding; Taiho: Research Funding; Kyowa-Kirin: Research Funding; Eizai: Research Funding; Bristol Myers Squibb: Research Funding; Chugai Pharmaceutical Inc, Roche: Ended employment in the past 24 months. Shirouchi:Symbio: Honoraria; Nippon Shinyaku: Honoraria; Chugai Pharmaceutical Inc, Roche: Honoraria; Meiji Seika Pharmaceutical: Honoraria; Janssen Pharmaceutical Inc: Honoraria; Bristol Myers Squibb: Honoraria; Sanofi: Honoraria. Yamauchi:Takeda: Research Funding; Genmab: Research Funding; Incyte: Research Funding; Ono: Research Funding. Terui:Symbio Pharmaceutical: Speakers Bureau; Bristl Myers Squibb: Speakers Bureau; Ono: Speakers Bureau; Eizai: Speakers Bureau; Chugai Pharmaceutical Inc, Roche: Speakers Bureau. Nojima:Chugai Pharmaceutical Inc, Roche: Consultancy. Maruyama:MSD: Honoraria, Research Funding; Nippon Shinyaku: Honoraria; Ono Pharmaceuticals: Honoraria, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Honoraria, Research Funding; Astellas: Research Funding; Otsuka: Research Funding; Janssen: Honoraria, Research Funding; Novartis: Research Funding; Celgene: Honoraria, Research Funding; Chugai Pharma: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Eizai: Honoraria, Research Funding; Amgen Astellas Biopharma: Research Funding; Taiho: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Zenyaku: Honoraria; SymBio Pharmaceuticals: Honoraria; AstraZeneca: Honoraria.